Bladder and bowel control problems include a range of embarrassing and potentially debilitating chronic health issues. Urinary urge incontinence (the involuntary leakage of urine accompanied by a sudden urge to urinate) and urgency-frequency (frequent urges to urinate), both symptoms of overactive bladder, are pervasive medical conditions with potentially disturbing social, sexual, interpersonal and professional consequences. Bowel incontinence (inability to control the bowels) is another devastating, socially isolating problem for which people are often reluctant to seek medical attention.
While people may delay seeking help for bladder and bowel control problems, they are highly treatable. Medtronic Bladder and Bowel Control Therapies (Sacral Neuromodulation) delivered by the InterStim® System may provide long-term relief for people suffering from symptoms of overactive bladder (urgency-frequency and urge incontinence), non-obstructive urinary retention or chronic bowel incontinence who have failed or are not candidates for more conservative treatments.
Medtronic Bladder Control Therapy delivered by the InterStim System is the first and only treatment option that uses an implantable neurostimulator to target the miscommunication between the bladder and the brain that may be causing symptoms. The implantable InterStim System provides mild electrical stimulation to help normalize this communication problem, which may otherwise cause leaks and frequent urges to urinate.
The InterStim System, the world’s first and only treatment option of its kind, was approved by the U.S. Food and Drug Administration (FDA) for the treatment of urinary urge incontinence in 1997, (non-obstructive) urinary retention and urinary urgency-frequency in 1999 and chronic fecal incontinence in 2011. InterStim Therapy is now used for both urinary control and bowel control in many geographies, including the United States, throughout Europe, Canada and Australia. To date, more than 175,000 people have received InterStim Therapy worldwide.
Treatment with the InterStim System involves three steps: an evaluation, a minimally invasive surgical implant, and ongoing post-implant follow-up appointments.
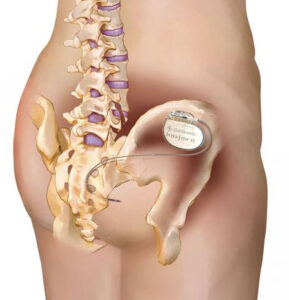
The process begins with all patients having an evaluation, which is used temporarily to determine if mild electrical stimulation of the sacral nerves may provide long-term relief for appropriate patients. The evaluation may be performed with the Verify® Evaluation System. During an in-office or outpatient procedure, a lead (thin wire) is placed near the tailbone, which is taped to the skin and connected to a small external device that sends mild electrical pulses to the sacral nerves using an external stimulator. The patient wears the external stimulator for 3-14 days to determine if he/she is likely to benefit from the InterStim System.
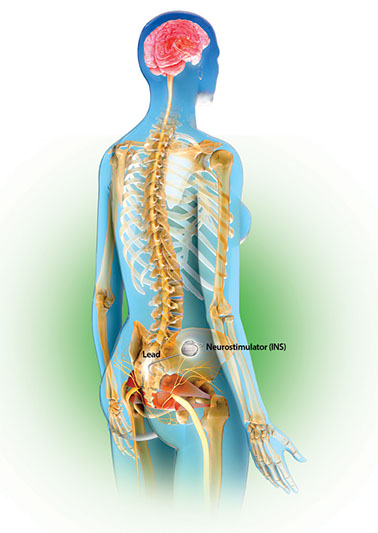
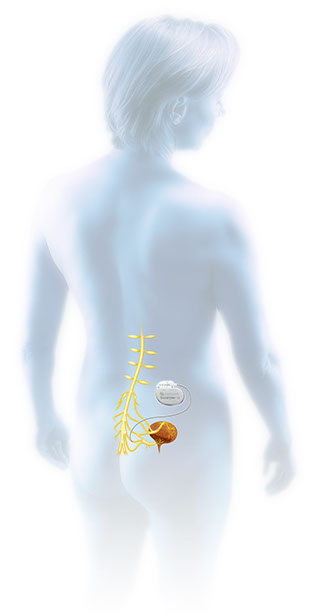
Following a successful evaluation, patients can choose to receive long-term therapy with the implantable InterStim System. A pacemaker-like device about the size of a stopwatch, called a neurostimulator, is connected to a permanent lead and placed under the skin in a minimally invasive outpatient procedure. The InterStim System then delivers mild electrical pulses to stimulate the sacral nerves and help manage bladder or bowel incontinence symptoms. The clinician adjusts the stimulation settings to optimize the therapy for each patient.
After the implant procedure, the patient can control the neurostimulation intensity within physician-set parameters using an external patient programmer that works like a remote control to turn the stimulation up and down or on and off. Follow-up examinations usually occur every six to 12 months to monitor for the therapy’s effectiveness. More visits may be required initially to achieve optimal settings.

Medtronic Bladder Control Therapy delivered by the InterStim System may be an option for patients with overactive bladder (urge incontinence and urgency-frequency) and non-obstructive urinary retention. Patients should consider Medtronic Bladder Control Therapy after they have tried other treatments such as fluid and diet modifications, medications and behavioral therapy and they have not worked successfully or could not be tolerated.*
Medtronic Bowel Control Therapy for the treatment of chronic bowel incontinence should be considered after patients with bowel control problems have tried other treatments such as medications and dietary modifications and they have not worked, or were not a candidate for these treatments.†
The InterStim System does not treat every type of bladder control or bowel control problem. For more information, please visit: www.medtronic.com
Complications can occur with the evaluation, including movement of the wire, technical problems with the device, and some temporary pain.
Implanting the InterStim System has risks similar to any surgical procedure, including swelling, bruising, bleeding, and infection. Complications associated with the InterStim System can include
Problems may be resolved with surgery, medical therapy such as drugs, or reprogramming. These events may also resolve over time. There is a possibility that some may remain unresolved. (See important safety information for additional information.) Please consult a doctor for additional information. This therapy is not for everyone. A prescription is required.
InterStim Therapy for Urinary Control: InterStim Therapy treats urinary retention (inability to completely empty the bladder) and the symptoms of overactive bladder, including urinary urge incontinence (leakage) and significant symptoms of urgency-frequency. It should be used after you have tried other treatments such as medications and behavioral therapy and they have not worked, or you could not tolerate them. InterStim Therapy for Urinary Control is not intended for patients with a urinary blockage.
Safety and effectiveness have not been established for pregnancy and delivery; patients under the age of 16; or for patients with neurological diseases such as multiple sclerosis.
InterStim Therapy for Bowel Control: InterStim Therapy treats chronic fecal incontinence (an accident or leaking involving stool). It should be used after you have tried other treatments such as medications and dietary modifications and they have not worked, or if you are not a candidate for them.
Safety and effectiveness have not been established for pregnancy and delivery; patients under the age of 18; or for patients with progressive, systemic neurological diseases.
InterStim Therapy for Urinary Control and for Bowel Control: You should have a successful trial assessment before receiving InterStim Therapy. You cannot have diathermy (deep heat treatment from electromagnetic energy) if you have an InterStim device.
In addition to risks related to surgery, complications can include pain at the implant sites, new pain, infection, lead (thin wire) movement/migration, device problems, interactions with certain other devices or diagnostic equipment such as MRI, undesirable changes in urinary or bowel function, and uncomfortable stimulation (sometimes described as a jolting or shocking feeling).
This therapy is not for everyone. Please consult your physician to decide whether InterStim Therapy is right for you. A prescription is required.
The Urology Center of Southern California is a single-specialty urology group which was founded in 1985.
Copyright © 2024 All Right Reserved By UCOSC.